Chemistry Portfolio
Metals
Where is metal extracted from?
Metals are found as minerals in ores. Ores are a type of rock that contain metals combined with several other elements known as minerals when put together. It is found in the earth’s crust. Minerals are a combination of metals. Examples of minerals are; Magnesium, Aluminium, Iron, Calcium, Potassium, Sodium and many others. The main element found in the crust is oxygen.
Gold is an element our ancestors used most. Gold is also a native mineral and so is not found mixed with other minerals because it is a nonreactive metal. And because at that time uor ancestors did not know how to separate the different elements due to their lack of chemical knowledge they felt it most suitable to use the element of gold metal.
How are metals extracted from the ore?

Most metals commonly extracted by reduction with carbon or electrolysis.
Extracting metals with Carbon
Metals such as zinc, iron and copper are present in ores as their oxides. Each of these oxides is heated with carbon to obtain the metal.
The metal oxide loses oxygen, and is therefore reduced. The carbon gains oxygen, and is therefore oxidised.
Using iron as an example:
iron oxide + carbon → iron + carbon dioxide
The source of carbon for this reduction is coke, obtained by heating coal in the absence of oxygen. Note that the iron is liquid when it is formed, due to the very high temperature at which the reaction takes place.Some metals, such as aluminium, are so reactive that their oxides cannot be reduced by carbon.
Carbon works as a reduction source because the element of Carbon is higher than many of the other metals in the reactivity series. And carbon can be used to extract these metals from their ores. It can be used to extract medium reactive metals.
Here below is the reactivity series in order of most reactive to least reactive metals:
K = Potassium
Na = Sodium
Ca = Calcium
Mg = Magnesium
Al = Aluminium
C = Carbon
Zn = Zinc
Fe = Iron
Sn = Tin
Pb = Lead
H = Helium
Cu = Copper
Ag = Silver
Au = Gold
Pt = Platinum
Another example:
Zinc oxide + carbon -> zinc + carbon monoxide
Here the oxide of zinc is reduced and the carbon is oxidised.

Image of Carbon
Electrolysis
Ionic compounds contain charged particles called ions. For example, copper(II) chloride contains positively charged copper ions and negatively charged chloride ions. Ionic substances can be broken down into the elements they are made from by electricity, in a process called electrolysis.
For electrolysis to work, the ions must be free to move. When an ionic compound is dissolved in water, or melts, the ions break free from the ionic lattice. These ions are then free to move.
For example, if electricity is passed through copper(II) chloride solution, the copper(II) chloride is broken down to form copper metal and chlorine gas.
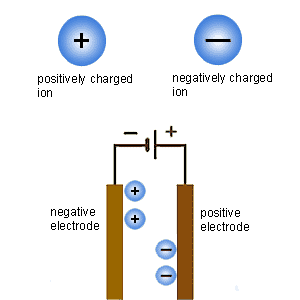
What happens in electrolysis. Positively charged ions move to the negatively charged electrode; negatively charged ions move to the positive electrode
There is a similar result if electricity is passed through molten copper(II) chloride.
The solution or molten ionic compound is called an electrolyte. The negative electrode is called the cathode, while the positive electrode is called the anode.
This is what happens during electrolysis:
Positively charged ions move to the negative electrode. Metal ions are positively charged, so metals are produced at the negative electrode (cathode).
Negatively charged ions move to the positive electrode. Non-metal ions, such as oxide ions and chloride ions, are negatively charged, so gases such as oxygen or chlorine are produced at the positive electrode (anode).
Taken from BBC bitesize... As I felt this was the best form of explanation
Process of Electrolysis
The life cycle of metal usage

Definitions
“Definitions of unknown words encountered in this unit”
Native: non reactive metals that are found as the element alone and these are called native minerals.
Reduction Reaction: Is when an element (commonly used; Carbon) replaces another element lower in the reactivity series so that the element extracted can be used for more useful purposes.
GLOBAL CONTEXT: For this unit it is "Globalisation and sustainability the interconnectedness of human-made systems and communities."