Chemistry Portfolio
BLOG
Organic Chemistry
“Is all about Carbon”
Global Context: Globalisation and Sustainability
Key Concept: Change
Inquiry: In order for structure and energy to continue driving change, finite fossil fuels will need to be replaced by renewable raw material (not processed).
Crude Oil

Crude oil is a think dark mixture found usually in the sea bed and it is made up of compounds called hydrocarbons. Many useful materials can be made using crude oil. Crude oil can be separated into different fractions using fractional distillation (heating up a furnace at a different temeprature at each fraction). Some of these fractions can go through a process called cracking which breaks the long chain hydrocarbons and these can then be used as fuels. This process also produces alkanes and alkenes, which can be used to make polymers.
Hydrocarbons
Most of the compounds in crude oil are hydrocarbons. This means that they only contain hydrogen and carbon atoms, joined together by chemical bonds. There are different types of hydrocarbon, but most of the ones in crude oil are alkanes.
Alkanes
The alkanes are a family of hydrocarbons that share the same general formula. This is:
CnH2n+2 - (subscript does not work on wix)
Alkenes
The alkenes are a family of hydrocarbons that share the same general formula. This is:
CnH2n
Alkynes
The alkynes are a family of hydrocarbons that share the same general formula. This is:
CnH2n-2
The below figure shows how the begininging of the word changes with the number of hydrocarbons in the molecule.
The table below explains the startings of the hydrocarbons more elaborately:
Fractional distillation
Fractional Distillation is a process whereby different sizes of hydrocarbons get serperated into catagories called fractions. A tall column is fitted above the mixture, with several condensers coming off at different heights. The column is hot at the bottom and cool at the top. Substances with high boiling points condense at the bottom and substances with low boiling points condense at the top. Like distillation, fractional distillation works because the different substances in the mixture have different boiling points.
Fractional distillation of crude oil
Because they have different boiling points, the substances in crude oil can be separated using fractional distillation. The crude oil is evaporated and its vapours allowed to condense at different temperatures in the fractionating column. Each fraction contains hydrocarbon molecules with a similar number of carbon atoms.
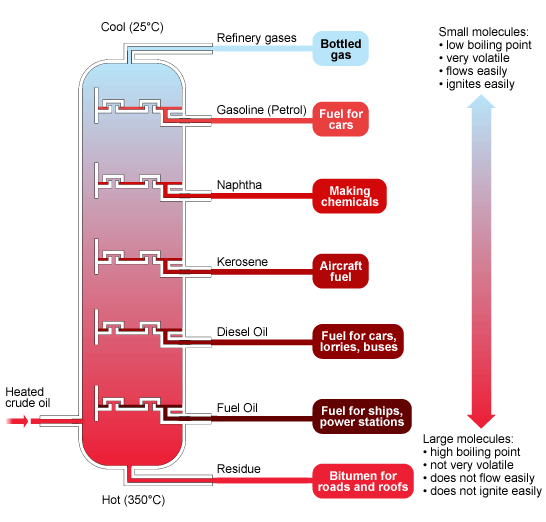
Oil fractions
The diagram below summarises the main fractions from crude oil and their uses, and the trends in properties. Note that the gases condense at the top of the column, the liquids in the middle and the solids stay at the bottom.
Cracking
Fuels made from oil mixtures containing large hydrocarbon molecules are not efficient. They do not flow easily and are difficult to ignite. Crude oil often contains too many large hydrocarbon molecules and not enough small hydrocarbon molecules to meet demand - this is where cracking comes in.
Cracking allows large hydrocarbon molecules to be broken down into smaller, more useful hydrocarbon molecules. Fractions containing large hydrocarbon molecules are vaporised and passed over a hot catalyst. This breaks chemical bonds in the molecules, and forms smaller hydrocarbon molecules.
Cracking is an example of a thermal decomposition reaction.
Go to the following website to view the reaction:
http://www.bbc.co.uk/schools/gcsebitesize/science/aqa_pre_2011/oils/polymersrev1.shtml
Polymers
Alkenes can be used to make polymers. Polymers are very large molecules made when many smaller molecules join together, end-to-end. The smaller molecules are called monomers. In general:
lots of monomer molecules → a polymer molecule
The animation shows how several chloroethene monomers can join end-to-end to make poly(chloroethene), which is also called PVC.
Visit http://www.bbc.co.uk/schools/gcsebitesize/science/aqa_pre_2011/oils/polymersrev5.shtml to view the animation
Alkenes can act as monomers because they have a double bond:
-
Ethene can polymerise to form poly(ethene), which is also called polythene.
-
Propene can polymerise to form poly(propene), which is also called polypropylene.
Different polymers have different properties, so they have different uses. The table below gives some examples.
Examples of polymers and their uses
polyetheneplastic -> bags and bottles
polypropene -> crates and ropes
polychloroethene -> water pipes and insulation on electricity cables
Visit this website for further information about polymers:
http://www.bbc.co.uk/schools/gcsebitesize/science/aqa_pre_2011/oils/polymersrev6.shtml
The page above is information direct from BBC Bitesize, Most of this text is unoriginal
Hydrocarbons and Alkanes